Assess for the Root Cause of Angina
Angiography is often used for diagnosing coronary artery disease, but is unable to accurately capture the plaque morphology and eccentricity of an epicardial stenosis. As a result, angiography alone cannot be used to objectively provide a comprehensive assessment of both the epicardial arteries and the microvasculature.1
A comprehensive physiology assessment enables interventional cardiologists (ICs) to:
- Go beyond the angiogram
- Diagnose and treat only functionally significant epicardial lesions at stress or rest2,3
- Diagnose microvascular disease in order to optimize patient management4
Watch physician experts discuss important factors to look for when measuring coronary physiology and explain why technique and the right tools matter.
Physiology-Guided Decision Making Leads to Better Outcomes5,6
The benefits of using coronary physiology include:
- Coronary angiography is not accurate in 34% of cases compared to Fractional Flow Reserve (FFR)7
- FFR physiology-guided percutaneous coronary intervention (PCI) reduces myocardial infarction (MI) compared to medical therapy alone8
- All No-Hyperemic Pressure Ratios (NHPR), including Resting Full-cycle Ratio (RFR), have similar outcomes and diagnostic performances6
- Adequate evaluation and optimal treatment of patients who have ischemia with no obstructive coronary artery disease (INOCA) can relieve patient symptoms, improving Quality of Life4
A Solution for Assessing Both the Epicardial Arteries and the Microvasculature9,10
With its pressure and temperature sensors, PressureWire™ X Guidewire has the capability to measure the entirety of the coronary circulation:9,10
- Pressure can be used to measure FFR, Pd/Pa, and RFR
- Temperature can be used to measure Index of Microcirculatory Resistance (IMR) and Coronary Flow Reserve (CFR) using thermodilution
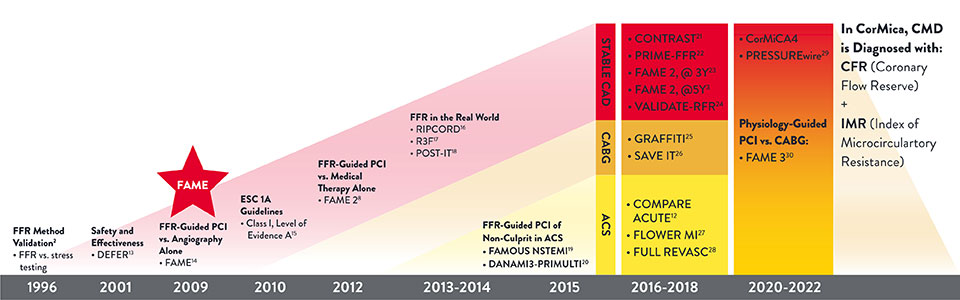
PressureWire™ X Guidewire has been extensively studied and clinically validated in both stable and acute coronary syndrome (ACS) patients.5,11-12
PressureWire™ Guidewire: 20 Years of Evidence
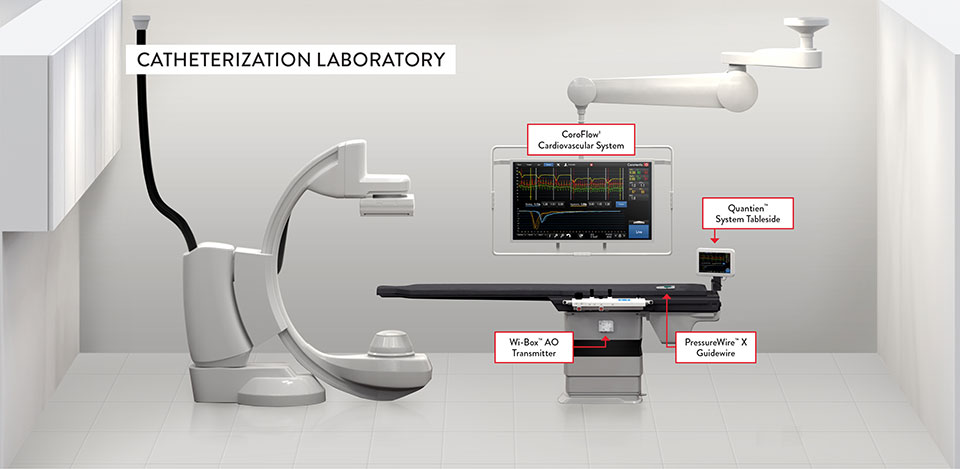
Multiple Connection Options Offer Stress Free Connectivity
PressureWire™ X Guidewire is the only9 wire with multiple connection options to eliminate clutter and meet your lab's needs.
References
- Topol EJ., et al. Our Preoccupation With Coronary Luminology. Circulation. 1995; 92(8): 2333-2342.
- Pijls N, et al. Measurement of Fractional Flow Reserve to Assess the functional severity of Coronary Artery Stenoses. N Eng J. 1996; 334 (1703-1708).
- Xaplanteris P, et al. FAME 2, Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018; 379:250-259
- Ford, TJ., et al. 1-year outcomes of angina management guided by invasive coronary function testing (CorMicA). J Am Coll Cardiol Intv. 2020; 13:33-45.
- Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up results of FAME study. J Am Coll Cardiol. 2010;56:177-184.
- Ahn JM, et al. IRIS FFR: prognostic performance of five resting pressure-derived indexes of coronary physiology. TCT 2018.
- Corcoran et al. Fractional Flow Reserve: A Clinical Perspective. Int J Cardiovasc Imaging. 2017; 33(7): 961-974.
- De Bruyne, B. et al. FFR Guided PCI versus Medical Therapy in Stable Coronary Disease. N Engl J Med. 2012; 367 (11): 991-1001.
- PressureWire™ X Guidewire Instructions for Use (IFU). Refer to IFU for additional information.
- CoroFlow‡ Cardiovascular System Instructions for Use (IFU). Refer to IFU for additional information.
- Zimmermann FM, et al. 2015: Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J. 2015;36(45):3182-3188. doi:10.1093/eurheartj/ehv452.
- Smits PC, Abdel-Wahab M, Neumann FJ, et al, on behalf of the Compare-Acute Investigators. Fractional flow reserve–guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234-1244.
- Bech GJW, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103:2928-2934.
- Tonino PA, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-224.
- Wijns W, et al. Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2010;31(20):2501-2555.
- Curzen N, et al. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain: the RIPCORD study. Circ Cardiovasc Interv. 2014;7(2):248-255.
- Van Belle E, et al. Outcome impact of coronary revascularization strategy reclassification with fractional flow reserve at time of diagnostic angiography: insights from a large French multicenter fractional flow reserve registry. Circulation. 2014;129(2):173-185.
- Baptista S, et al. Impact of Routine Fractional Flow Reserve Evaluation During Coronary Angiography on Management Strategy and Clinical Outcome. One-year results of the POST-IT Multicenter Registry. Circ Cardiovasc Interv. 2016;9:e003288.
- Layland J, et al. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non-ST-segment elevation myocardial infarction: the British Heart Foundation FAMOUS–NSTEMI randomized trial. Eur Heart J. 2015;36(2):100-111.
- Engstrøm T, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-671.
- Nishi T, et al. Influence of contrast media dose and osmolality on the diagnostic performance of contrast fractional flow reserve. Circ Cardiovasc Interv. 2017;10:e004985.
- Van Belle E, et al. Impact of routine fractional flow reserve on management decision and 1-year clinical outcome of patients with acute coronary syndromes: PRIME-FFR (insights from the POST-IT [POrtuguese Study on The Evaluation of FFR-guIded Treatment of coronary disease] and R3F [French FFR Registry] Integrated MulticEnter registries - implementation of FFR [fractional flow reserve] in routine practice). Circ Cardiovasc Interv. 2017;10:e004296.
- Fearon WF, et al. Clinical outcomes and cost-effectiveness of fractional flow reserve–guided percutaneous coronary intervention in patients with stable coronary artery disease: three-year follow-up of the FAME 2 Trial. Circulation. 2018;137:480-487.
- Svanerud J, et al. Validation of a novel non-hyperaemic index of coronary artery stenosis severity: the Resting Full-cycle Ratio (VALIDATE RFR) study. EuroIntervention. 2018; 14:806-814.
- Toth GG, De Bruyne B, Kala P, et al. Graft patency after FFR-guided versus angiography-guided coronary artery bypass grafting: the GRAFFITI trial. EuroIntervention. 2019;15(11):e999-e1005.
- Ramos R, Batista S, Raposo L, et al. Strategies for revascularization in patients undergoing heart valve surgery with concomitant coronary artery disease (SAVE-IT). Website accessed on 9/20/2022: https://clinicaltrials.gov/ct2/show/NCT02173860.
- Puymirat E, et al. FLOW Evaluation to guide Revascularization in multi-vessel ST-elevation Myocardial Infarction (FLOWER-MI). Website accessed on 9/20/2022: https://clinicaltrials.gov/ct2/show/NCT02943954.
- Bohm F, et al. Ffr-gUidance for compLete Non-cuLprit REVASCularization (FULL REVASC). Website accessed on 9/20/2022: https://clinicaltrials.gov/ct2/show/NCT02862119.
- Schampaert E, Kumar G, Achenbach S, et al. A global registry of fractional flow reserve (FFR)-guided management during routine care: study design, baseline characteristics and outcomes of invasive management. Catheter Cardiovasc Interv. Published online ahead of print, March 14, 2020.
- Pijls NHJ, et al. A comparison of fractional flow reserve-guided percutaneous coronary intervention and coronary artery bypass graft surgery in patients with multivessel coronary artery disease (FAME 3). NCT02100722. Website accessed on 9/22/2022: https://clinicaltrials.gov/ct2/show/NCT02100722
MAT-2208166 v1.0
