*Caution: The Amulet™360 Left Atrial Appendage Occluder* is an INVESTIGATIONAL DEVICE. LIMITED BY LAW TO INVESTIGATIONAL USE ONLY. This product is not available for sale in any geography.
First results from the VERITAS Study were presented at AF Symposium 2026, providing the earliest clinical evidence evaluating the Amulet™ 360 LAAO* in patients with nonvalvular AF. The findings show that both primary endpoints were met with statistically significant performance, supported by independent core lab review.
Study Outcomes1
Early findings from the VERITAS Study were presented at the 2026 AF Symposium in Boston, providing the first data on the Amulet™ 360 LAAO* device.
0%
Primary Safety Endpoint*
p < 0.0001 (performance goal: 3.75%)
100%
Primary Effectiveness Endpoint*
p < 0.0001 (performance goal: 96.6%)
100%
Clinically relevant closure (no PDL > 3mm identified
93.9%
Complete closure (0 mm leak) identified
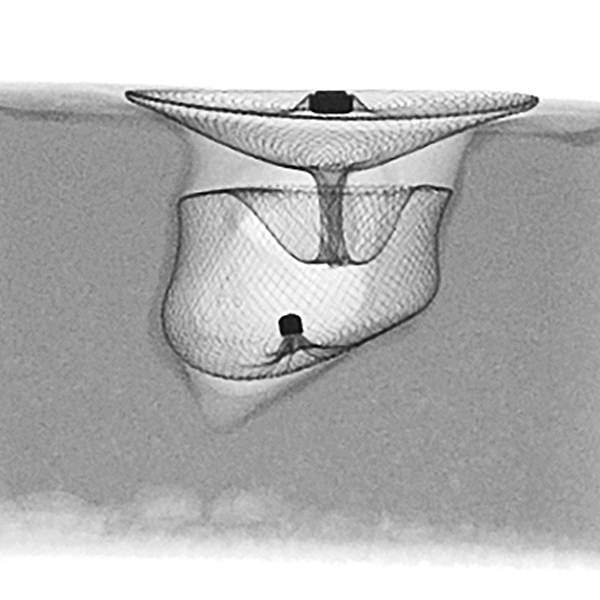
Initial procedural metrics.
99.8%
implant success
88%
of subjects implanted with a single device attempt
0.5%
pericardial effusion requiring drainage
0%
device embolization
Questions? Reach out to: EPScience@abbott.com
1. Nair D. Early outcomes with a next-generation dual-seal device for left atrial appendage closure: Results from the VERITAS Amulet 360 Pivotal study. Presented at: AF Symposium; February 6, 2026; Boston, MA, USA.
CAUTION: Product(s) intended for use by or under the direction of a physician. Prior to use, reference to the Instructions for Use, inside the product carton (when available) or at https://www.eifu.abbott/for more detailed information on Indications, Contraindications, Warnings, Precautions and Adverse Events.
MAT-2600761 v1.0
