XIENCE™ Stent Evidence
XIENCE™ Stent is the DES that delivers consistently outstanding short- and long-term patient outcomes.2
In fact XIENCE™ Stent consistently has the lowest rate of late and very late stent thrombosis (ST)—not only a year but a decade after the intervention—providing evidence that the XIENCE™ Stent's unparalleled patient outcomes last far beyond the intervention.1
Long-Term Patient Data
XIENCE™ Stent’s Consistently Low Rates of Late and Very Late Stent Thrombosis1
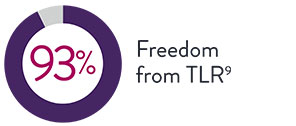
7-Year TLR Data
Long-term restenosis rates are also low, as shown by freedom from clinically driven target lesion revascularisation (CD-TLR) at 7 years.9
Optimal Device Success
XIENCE™ Stent data shows consistent device success—and in all types of patients and lesions.

Improved Quality of Life with XIENCE™ Stent11
XIENCE™ Stent has been shown to improve the quality of life of left main patients out to 3 years with 81% of patients reporting that they were angina free.
See the long-term results in complex patients treated with XIENCE™ Stent.
Improved QOL at 3 Years11

References
- Zanchin C, et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333. Palmerini T, et al. Lancet. 2013;379:1393-1402. Bangalore S, et al. Circulation. 2012;125:2873-2891. Bangalore S, et al. Circ Cardiovasc Interv. 2013;6(6):378-390. Pilgrim T, et al. Lancet. 2014;384:2111-2122. Pilgrim T, et al. Lancet. 2018;392:737-746. Data on file at Abbott.
- Zanchin C. et al. JACC Cardiovasc Interv. 2019;12(17):1665-1675. Serruys P, et al. N Engl J Med. 2010;363:136-146. Shiomi H, et al. JACC Cardiovasc Interv. 2019;12:637-647. Kufner S, et al. Circulation. 2019:139(3):325-333.
- Mehran R, et al. TCT Connect - XIENCE 28/90.
- Von Birgele C, et al. J Am Coll Cardiol. 2012;59(15):1350-1361.
- Van Geuns RJ, et al. TCT 2019, IDEAL-LM.
- Chevalier B, et al. EuroIntervention. 2018;13(13):1561-1564.
- Gao R, et al. TCT 2019, ABSORB China.
- Kufner S, et al. Circulation. 2019:139(3):325-333.
- Shiomi H, et al. JACC Cardiovasc Interv. 2019;12(7):637-647.
- Saito S, et al. Eur Heart J. 2014;35:2021-2031. Saito S, et al. EuroIntervention. 2019;15(11):e1006-e1013. Natsuaki M, et al. J Am Coll Cardiol. 2013;62(3):181-190. Stone G, et al. Lancet. 2018:392:1530-40.
Note: 99% was the lowest device success rate. - Baron SJ, et al. J Am Coll Cardiol. 2017;70(25):3113-3122.
MAT-2413719 V1.0

