Clinical Evidence
MOMENTUM 3 is the largest LVAD trial ever conducted demonstrating excellent survival and safety outcomes with HeartMate 3™ LVAD.*1 For the first time in LVAD history, 5-year data is available from this prospective randomized LVAD trial.2
HEARTMATE 3™ LVAD, a proven long-term, life-extending therapy for patients with advanced heart failure
Median survival
exceeding
5 years

58.4%
survival at
5 years
Life-prolonging therapy in a
patient population who
otherwise would not be
expected to survive beyond 9
months2,3**
Superiority of the HeartMate
3 LVAD driven by a reduction
in hemocompatibility- related
adverse events2
Survival comparable to
higher-risk transplant
patients2,4
Based on published data from multicenter experience and separate studies, which may involve different patient populations and other variables. Not a head-to-head comparison. Data presented for informational purposes only.
ELEVATE Registry
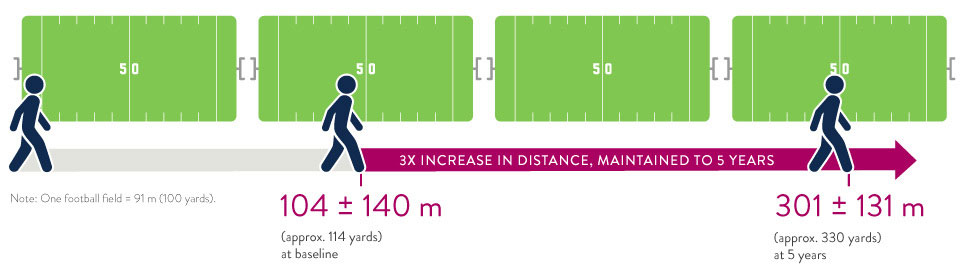
The ELEVATE Registry evaluated the real-world experience of the HeartMate 3 LVAD in a post-approval setting.5 5-year extended follow-up showed significant increase in 6-minute walk distance.
Significant Increase in 6-Minute Walk Distance5
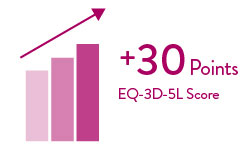
Quality-of-life improved significantly and was sustained through 5 years 5
As measured by the EuroQoL-5 Dimensions-5 Levels (EQ-5D-5L) questionnaire
Improved Safety Profile5
Stroke
10.8% in first 2 years
3.6% in years 2-5
Thrombosis
1.1% at 5 years
All major event types were reduced in the 2-5 years follow-up period compared to 0-2 years, especially hemocompatiblity-related adverse events.
This device is commercially available for use in select international markets.
*HeartMate 3 LVAD demonstrated superiority in event-free survival (primary endpoint) in the MOMENTUM 3 trial compared to HeartMate II™ LVAD.
**Patients on inotropes who did not receive a transplant or left ventricular assist device.
References
- Mehra M, Uriel N, Naka Y, et al. A Fully Magnetically Levitated Left Ventricular Assist Device-Final Report. N Engl J Med. 2019;380:1618-1627.
- Mehra MR, Goldstein DJ, Cleveland JC, et al. Five-Year Outcomes in Patients With Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices in the MOMENTUM 3 Randomized Trial. JAMA. September 8, 2022. doi:10.1001/jama.2022.16197
- Hashim T, Sanam K, Revilla-Martinez M, et al. Clinical Characteristics and Outcomes of Intravenous Inotropic Therapy in Advanced Heart Failure. Circ Heart Fail. 2015;8(5):880-886.
- Kilic et al. Factors associated with 5-year survival in older heart transplant recipients. J Thorac Cardiovasc Surg 2012;143:468-74.
- Schmitto JD, Shaw S, Garbade J, et al. Long-Term Results in Real World Patients Treated with HeartMate 3 LVAD for Advanced Heart Failure: Data from the ELEVATE Registry. Presented at: European Association for Cardio-Thoracic Surgery (EACTS) Annual Meeting; October 8, 2022; Milan, Italy.
MAT-2105164 v3.0
