MOMENTUM 3 is the largest LVAD trial ever conducted demonstrating excellent survival and safety outcomes with HeartMate 3™ LVAD.*1 For the first time in LVAD history, 5-year data is available from this prospective randomized LVAD trial.2

Based on published data from multicenter experience and separate studies, which may involve different patient populations and other variables. Not a head-to-head comparison. Data presented for informational purposes only.
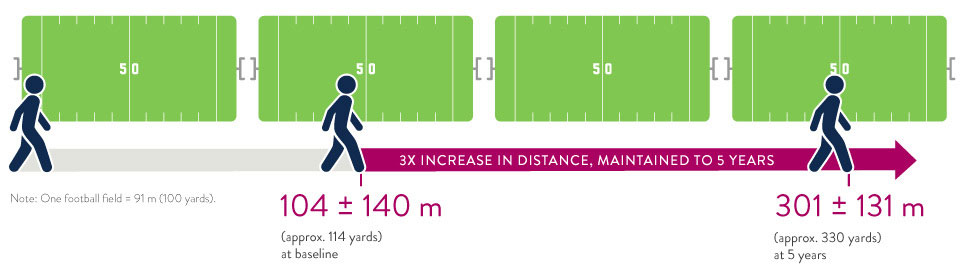
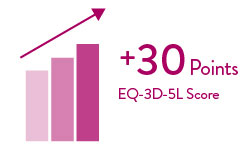
The ELEVATE Registry evaluated the real-world experience of the HeartMate 3 LVAD in a post-approval setting.5 5-year extended follow-up showed significant increase in 6-minute walk distance.
*HeartMate 3 LVAD demonstrated superiority in event-free survival (primary endpoint) in the MOMENTUM 3 trial compared to HeartMate II™ LVAD.
**Patients on inotropes who did not receive a transplant or left ventricular assist device.
MAT-2105164 v3.0
You are about to enter an Abbott country- or region-specific website.
Please be aware that the website you have requested is intended for the residents of a particular country or countries, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions
Do you wish to continue and enter this website?
MAT-2305078 v1.0