These workhorse peripheral guide wires offer different support and penetration power to access and penetrate soft to heavily calcified lesions.
The Power to Connect
Hi-Torque Connect™ Guide Wire

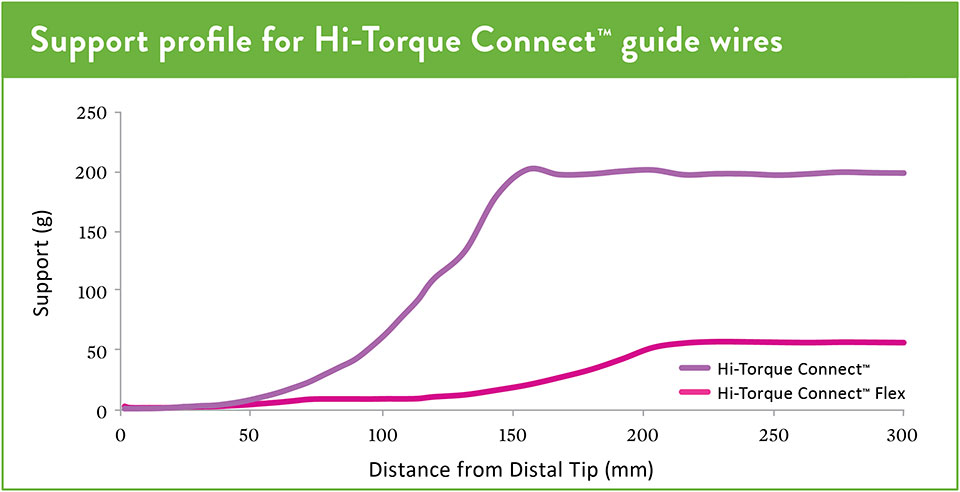
Supportive—for focal stenosis. This workhorse guide wire has a supportive body, soft tip, and polymer cover.
4.4 g tip load* for soft to moderately calcified lesions1
Hi-Torque Connect™ Flex Guide Wire
.png)
Trackable—for tortuous anatomy. This workhorse guide wire has a flexible body, stiff tip, and polymer cover.
14.9 g tip load* for moderately calcified lesions1
Ordering information for Hi-Torque Connect™ 250 T Guide Wires can be found in the Specialty Guide Wire Section.
Options for Hi-Torque Connect™ and Hi-Torque Connect™ Flex Guide Wires
Support
Choose varying levels of support to provide options for lesion crossing in straight to tortuous anatomies
Choice
Select either 4.4 g or 14.9 g tip load*, and corresponding penetration powers, to enhance crossing the lesion in either soft or moderate calcification
Control
The core-to-tip guide wire design enables outstanding torque performance and control
Ordering information for Hi-Torque Connect™ 250 T Guide Wires can be found in the Specialty Guide Wire Section.
Ordering Information
| Product | Part Number | Diameter | Length | Tip Load* | Tip Style |
|---|---|---|---|---|---|
| Hi-Torque Connect™ | 1012587 | 0.018 in | 145 cm | 4.4 g | Core-To-Tip |
| Hi-Torque Connect™ | 1012588 | 0.018 in | 195 cm | 4.4 g | Core-To-Tip |
| Hi-Torque Connect™ | 1012589 | 0.018 in | 300 cm | 4.4 g | Core-To-Tip |
| Hi-Torque Connect™ Flex | 1012590 | 0.018 in | 145 cm | 14.9 g | Core-To-Tip |
| Hi-Torque Connect™ Flex | 1012591 | 0.018 in | 195 cm | 14.9 g | Core-To-Tip |
| Hi-Torque Connect™ Flex | 1012592 | 0.018 in | 300 cm | 14.9 g | Core-To-Tip |
*Data on file at Abbott.
References
- Tóth GG, Yamane M, Heyndrickx GR. Heart. 2015;101:645–652.
MAT-2409312 v1.0

