The Clinically proven Tendril™ STS pacing lead has been enhanced with the addition of SurGrip™ technology, providing a new level of secure fixation and advanced lead protection.
The Tendril STS with SurGrip technology provides a reliable, safe and 'No Wait' MRI-ready solution with the performance you can depend on.

in cut-through resistance1
in lead crush resistance1
in lead slip force resistance (tied to 4 lbs.)1
Tendril's reliable performance delivers confidence through actively monitoring long-term data.
implants worldwide2
of implants2
lead survival at 134 months2
actively monitored leads2
Minimize lead abrasion with Optim™ lead insulation, a unique hybrid material that:
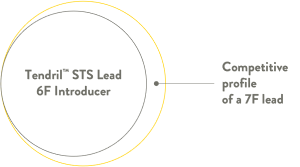
Safer 6-French design features a thing profile pacing lead designed for flexibility and ease of handling for a safer and easier venous introduction.4
Thin lead body for safer and easier venous introduction. Ease implantation with the small diameter lead, compatible with a 6F introducer.
Soft silicone tip allows for more compliance at the lead-endocardium interface and enhances implant safety.
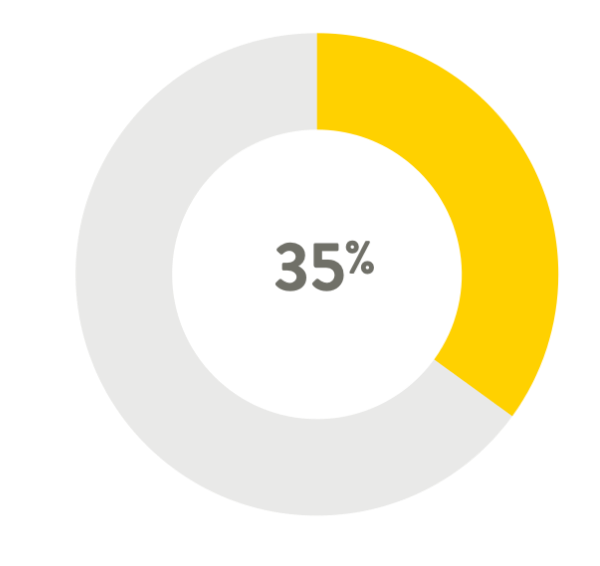
An increase in lead tip surface
area of 35%4
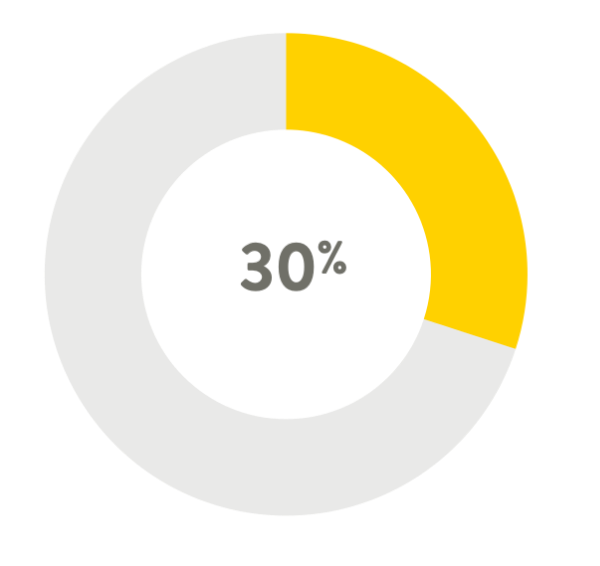
A tip pressure reduction of 30% (mean)4
Ensures patients can get the care they need, when they need it, with MRI-ready portfolio solutions.
versus 42 days for competitive systems5-7
both MRI-ready systems**
of MRI scans are urgent or emergent8
Implanted devices and lead combinations are MR conditional.
Tendril™ STS 2088TC – 46, 52, 58 cm
Tendril MRI™ LPA1200M — 46, 52, 58 cm

Tendril STS 2088TC – 46, 52 cm
Tendril MRI LPA1200M – 46, 52 cm
Durata™ 7120Q – 58, 65 cm
Durata 7122Q – 58, 65 cm
Optisure™ LDA220Q – 58, 65 cm
Optisure™ LDA210Q – 58, 65 cm

Tendril STS 2088TC – 46, 52 cm
Tendril MRI LPA1200M – 46, 52 cm
Durata™ 7120Q – 58, 65 cm
Durata 7122Q – 58, 65 cm
Optisure™ LDA220Q – 58, 65 cm
Optisure™ LDA210Q – 58, 65 cm

Tendril STS 2088TC – 46, 52, 58 cm
Quartet™ 1456Q/1457Q1458Q/1458QL – 86 cm
**PM3562 only

Tendril STS 2088TC – 46, 52 cm
Tendril MRI LPA1200M – 46, 52 cm
Durata™ 7120Q – 58, 65 cm
Durata 7122Q – 58, 65 cm
Optisure™ LDA220Q – 58, 65 cm
Optisure™ LDA210Q – 58, 65 cm
Quartet™ 1456Q/1457Q1458Q/1458QL – 86 cm

Tendril STS 2088TC – 46, 52 cm
Tendril MRI LPA1200M – 46, 52 cm
Durata™ 7120Q – 58, 65 cm
Durata 7122Q – 58, 65 cm
Optisure™ LDA220Q – 58, 65 cm
Optisure™ LDA210Q – 58, 65 cm
Quartet™ 1456Q/1457Q1458Q/1458QL – 86 cm

| Magnet (Tesla) | 1.5T, 3T |
|---|---|
| Scan Type | Full-body |
| Scanner Mode | Normal Operating Mode |
*No miminum length of time requirement between implant and MRI scan. Stability of pacing capture thresholds is required prior to MRI scan.
**For additional information about specific MR Conditional and lead model numbers, including warnings, precautions, adverse conditions to MRI scanning and potential adverse events, please refer to the Abbott MRI-Ready Systems Manual or check our MRI Ready resources.
MAT-2011130 v3.0
STAY CONNECTED