Embolic Protection Devices can add a layer of protection during endovascular procedures in the lower extremities or carotids. In the lower extremities, downstream complications can be minimized and in the carotids, the stroke rate can be reduced with the use of EPDs.1
Three trials evaluated carotid stenting with the Emboshield NAV6™ EPS and/or previous generation Abbott products.
| Summary of Clinical Trial Data for Emboshield NAV6™ Embolic Protection System | |||
|---|---|---|---|
| ACT I2 (n = 1,453; carotid stenting n = 1,089) Asymptomatic patients at standard risk for CEA | CHOICE3 (n = 17,925) Symptomatic and asymptomatic patients at high risk for CEA | PROTECT4 (n = 220) Symptomatic and asymptomatic patients at high risk for CEA | |
| DSMI (30 Days) | 3.3% | 4.2% | 2.3% |
| DS (30 Days) | 2.9% | 3.8% | 1.8% |
| Death or Major Stroke (30 Days) | 0.6% | 1.4% | 0.5% |
| Freedom from Ipsilateral Stroke | 97.8% (30 Days – 5 Years) | Not evaluated | 98.8% (31 Days – 3 Years) |
DS = Death or stroke | DSMI = Death, stroke, or myocardial infarction | CEA = Carotid endarterectomy
The primary aim of this prospective, multicenter trial was to compare the outcomes of stenting with embolic protection vs carotid endarterectomy.
The 1,453 patients were randomly assigned to the stenting group (n = 1,089) or the CEA group (n = 364), all of whom met the following criteria:
| ACT I Trial | ||
|---|---|---|
| Carotid Artery Stenting (CAS) | Carotid Endarterectomy | |
| Primary composite endpoint: DSMI (at 30 days) and ipsilateral stroke (31 days - 1 year) | 3.8% | 3.4% |
| Freedom from ipsilateral stroke (31 days - 5 years) | 97.8% | 97.3% |
| Freedom from clinically driven revascularization (5 years, p = 0.05) | 98.4% | 96.7% |
| 5 year survival | 87.1% | 89.4% |
The authors concluded that:
With 17,925 patients evaluated, the CHOICE trial represents the largest prospective, single-arm, adjudicated, multicenter CAS data set to date. The CHOICE study also provided additional post-market surveillance of RX Acculink™ Carotid Stent System and Abbott’s embolic protection systems.
Patient criteria included:
There were other notable aspects of the patient population:
The 30-day findings included:
| CHOICE Trial | ||
|---|---|---|
| All Patients (n = 17,925) | Patients Age < 80 (n = 13,868) | |
| DSMI | 4.2% | 3.4% |
| DS | 3.8% | 3.0% |
| Death or major stroke | 1.4% | 1.1% |
The investigators concluded that CAS is a viable option for patients at high risk for CEA. In addition, favorable outcomes were observed in patients < 80 years of age.
Investigators undertook the PROTECT trial (n = 220) in an effort to evaluate the outcomes with improved device technology.
The PROTECT trial included only patients at high surgical risk for CEA, and severe stenosis:
| PROTECT Trial | |
|---|---|
| DS (30 days) | 1.8% |
| DSMI (30 days) | 2.3% |
| Death or Major Stroke (30 days) | 0.5% |
| Freedom from Ipsilateral Stroke (31 days – 3 years) | 98.8% |
These data reveal improved outcomes compared to earlier high-risk CAS trials.
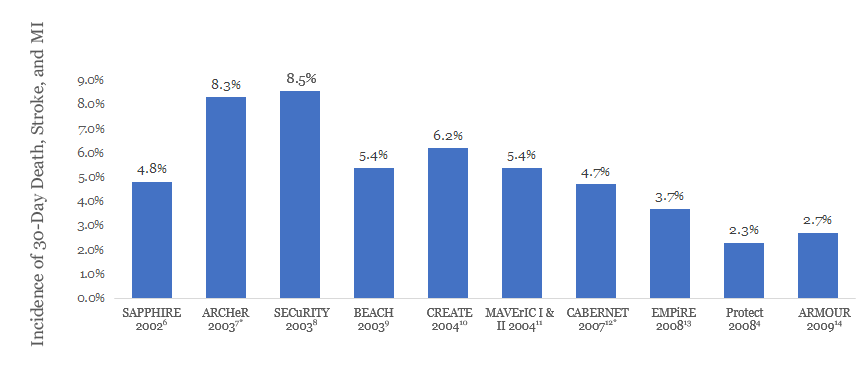
NOTE: Results from clinical trials are not directly comparable. Information provided for educational purposes only.
The Lower Extremity Real World Data Analysis is a prospective analysis of real-world data collected from 162 patients receiving embolic protection with Emboshield NAV6™ during atherectomy for femoral popliteal lesions in real-world clinical practice.
The primary analysis outcome was freedom from major adverse events (MAE) at 30 days, which was compared against a performance goal derived from MAE rates of similar devices used in the same anatomy.
Patients
Patient criteria:
Patients were treated per standard of care and choice of atherectomy, atherectomy device, use of filter, and filter type was at the discretion of the operator.
Findings
All Emboshield NAV6™ filters were delivered successfully, and there were no reports of device malfunction. One complication, a perforation caused by migration of the wire in the filter, occurred. The complication was treated without sequelae. Macroemboli was present in approximately 60% of cases, and filter overflow occurred in 10.5% of cases.
The 30-day freedom from MAE rate was 92.0%, in which the lower limit of the two-sided 95% confidence interval was 86.7%, meeting the pre-specified PG of 83%.
| Freedom from Major Adverse Events Rate vs. Performance Goal | ||
|---|---|---|
| Primary Endpoint | Performance Goal | Emboshield NAV6™ EPS (n = 162) |
| Freedom from MAE [95% Confidence Interval]1 | 83% | 92.0% (149/162) [86.7%, 95.7%] |
* 30-day death, stroke, and MI plus late (31–365 days) ipsilateral stroke
MAT-2200415 v1.0
You are about to enter an Abbott country- or region-specific website.
Please be aware that the website you have requested is intended for the residents of a particular country or countries, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions
Do you wish to continue and enter this website?
MAT-2305078 v1.0