Khalsa et al., JVIR. 2020.
Objective
To study the efficacy of the JETi™ Peripheral Thrombectomy System in treating acute venous thrombosis.
Methods
Retrospective review of 40 total procedures in 30 patients with acute lower extremity venous thrombosis (25 iliocaval, 20 iliofemoral and 15 femoropopliteal segments).
Primary Endpoints
Technical Success Rate defined as reestablishment of inline flow.
Additional Endpoints
Percent thrombus removal, total thrombolytic dose, total procedural time, overnight thrombolysis post procedure, ICU length of stay, total hospital length of stay, procedure related complications, mean symptom duration and venous segments treated.
JETi™ Peripheral Thrombectomy System achieved a 93% overall technical success rate with 77% of patients successfully treated in a single-session. Patients treated with JETi™ in a single-session required less TPA and shorter ICU stays compared to those treated with overnight CDT.
| Single-Session JETi™ Thrombectomy (N = 23/30) | Overall JETi™ Thrombectomy* (N = 30/30) | |
|---|---|---|
| Mean ICU Length of Stay | 1.1 days | 1.6 days |
| Mean TPA Dose | 3.1 mg | 7.8 mg |
| Mean Thrombus Removal | 81.2% | 74.0% |
*Data includes 7 patients requiring overnight CDT.
Restoration of inline flow was successfully achieved in 77% of patients in a single session.
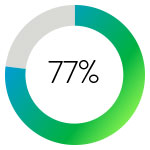
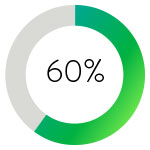
Single-session restoration of flow was associated with shorter ICU stays and a 60% lower dose of TPA when compared with overnight CDT.
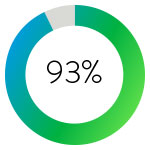
Technical success was achieved in 93% of patients treated with the JETi™ Thrombectomy System.
Khalsa et al., JVIR. 2020.
MAT-2201499 v1.0

The JETi™ Hydrodynamic Thrombectomy System is intended to remove/aspirate fluid and break-up soft emboli and thrombus from the peripheral vasculature and to sub-selectively infuse/deliver diagnostics or therapeutics with or without vessel occlusion.
The JETi™ Hydrodynamic Thrombectomy System is contraindicated for use in:
| Fluid | Maximum Recommended Fluid Delivery Flow Rate |
|---|---|
| Saline | 4.0 mL/s |
| 60% Ionic Contrast Media | 1.8 mL/s |
Potential adverse events include, but are not limited to:
JETi™ is a trademark of Walk Vascular. Walk Vascular is a subsidiary of the Abbott Group of Companies.
Manufactured by Walk Vascular LLC 17171 Daimler Street, Irvine CA, 92614 USA
MAT-2116195 v3.0
STAY CONNECTED