OPTIS™ Integrated Next Imaging System and OPTIS™ Mobile Next Imaging System are the newest generation of Abbott’s imaging systems that are available with Ultreon 1.0 Software. Streamlined and intuitive, Ultreon™ 1.0 Software gives better insights to optimize patient outcomes through automation and an improved workflow.1-4 The OPTIS™ Next Systems use high-powered processors that support AI technology for faster information display and workflow efficiency.1
OPTIS™ Integrated Next System is installed in a single cath lab; it's always on and always ready to perform intravascular imaging and coronary physiology.
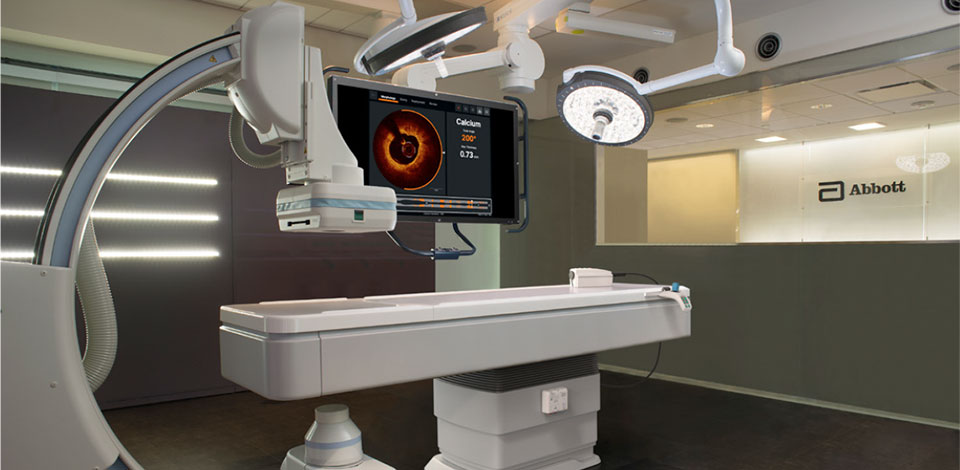
The OPTIS™ Integrated Next System is the latest integrated imaging system that supports Ultreon™ 1.0 Software. It is recommended for imaging and physiology needs, for busy cath labs and high PCI volume centers when the availability of OCT and/or coronary physiology is always needed without advanced arrangements.

OPTIS™ Mobile Next Imaging System is a transportable system designed for use in multiple cath labs with easy pre-installed connections.
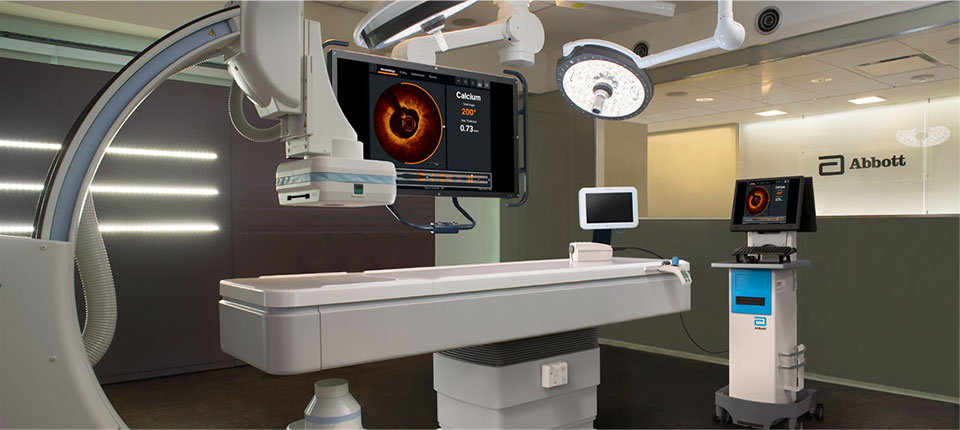
The OPTIS™ Mobile Next System provides the same functionality as the OPTIS™ Integrated Next System, but provides mobile system workflow via an easily transportable cart to serve multiple cath labs.
The OPTIS™ Mobile Next Imaging System is the latest mobile imaging system that supports Ultreon™ 1.0 Software. It is recommended for multiple cath labs that require flexibility or for busy cath labs that already have an OPTIS™ Integrated Next Imaging System and need an additional system as a back up.
OPTIS™ Next Imaging Systems IFU. Refer to Instructions For Use (IFU) for additional information.
MAT-2106765 v1.0

The Ultreon™ 1.0 Software is intended to be used only with compatible OPTIS™ Next Imaging Systems. The OPTIS™ Next Imaging System with a compatible Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure. The OPTIS™ Next Imaging System is intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment to determine if therapeutic intervention is indicated.
Contraindications: Use of the Ultreon™ 1.0 Software is contraindicated where introduction of any catheter would constitute a threat to patient safety.
Contraindications include:
Complications: The risks involved in vascular imaging include those associated with all catheterization procedures. The following complications may occur as a consequence of intravascular imaging and may necessitate additional medical treatment including surgical intervention.
Warnings:
Precautions:
MAT-2104193 v3.0
STAY CONNECTED