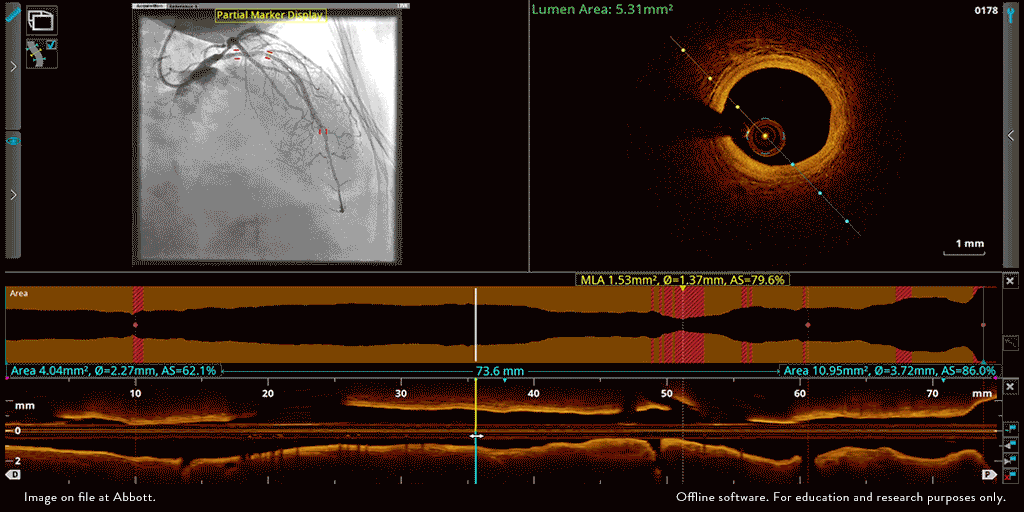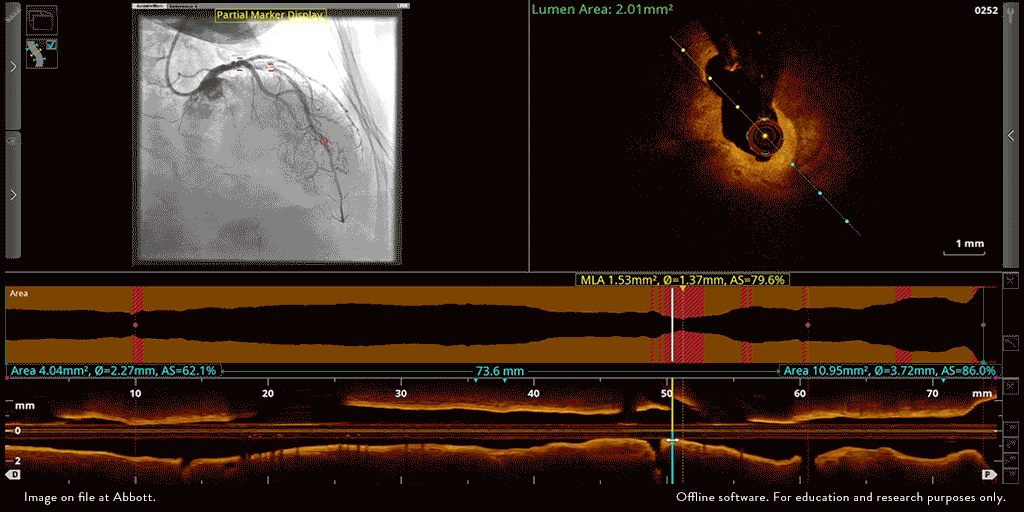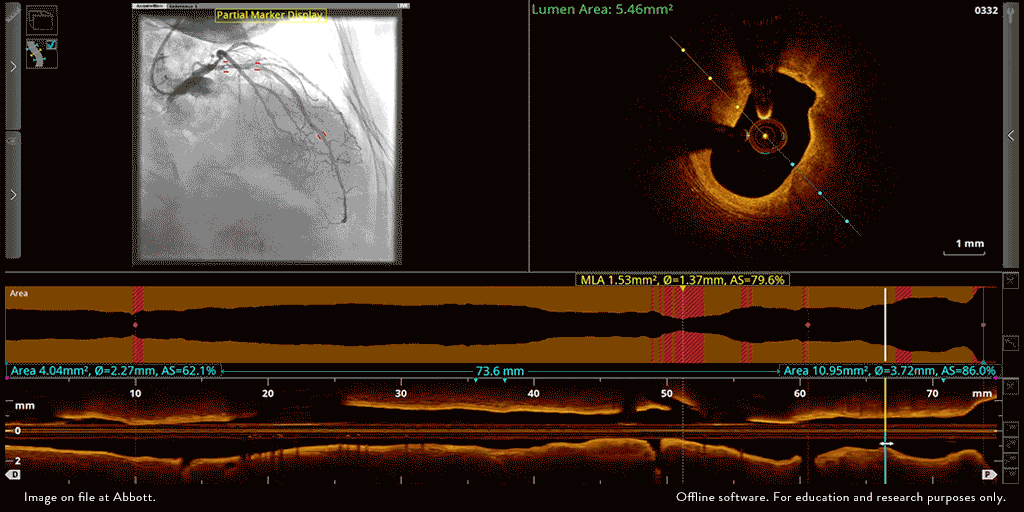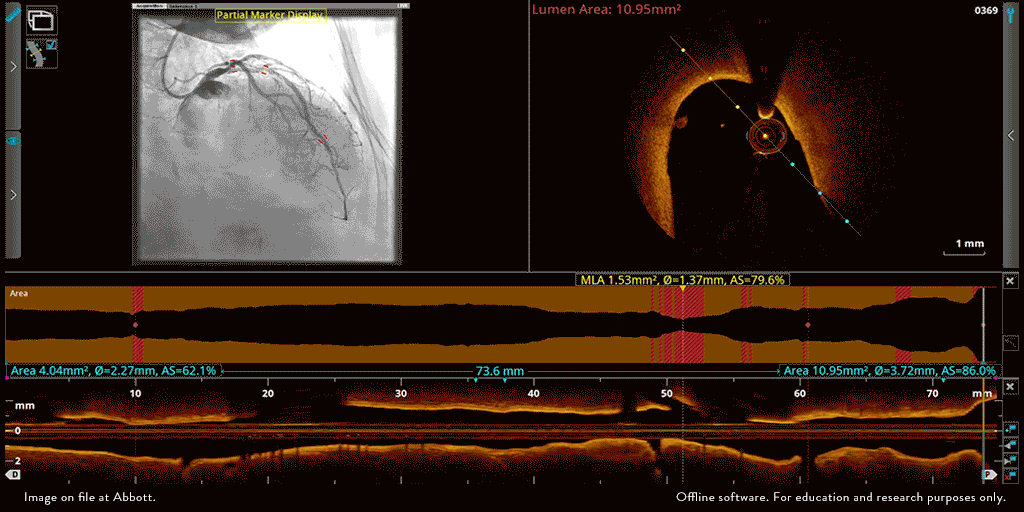
Abbott’s OPTIS™ Imaging Systems are easily integrated with the cath lab systems.
The OPTIS™ Imaging System displays coronary physiology (FFR/RFR*) measurement using a wireless PressureWire™ X Guidewire and an external aortic pressure (AO) transducer. Aortic pressure signal is sent from an AO transducer to Wi-Box™ AO Transmitter and wirelessly transmitted to the OPTIS™ Imaging System.1

Wi-Box™ AO Transmitter is a small device easily installed by mounting on the base of the cath lab table. No cables to connect to OPTIS™ Imaging System, no clutter, no interference with the flow of the PCI procedure. Wi-Box™ AO Transmitter sends the AO pressure signal wirelessly to the OPTIS™ Imaging System and the output is displayed on the existing cath lab monitors.
OPTIS™ Imaging Systems implement several DICOM services for exchanging information and images. These systems allow for the querying of patient information from a network storage device, as well as the storing of acquired OCT images and physiology images, as “secondary capture” images, to the network storage device or CD/DVD.
To view DICOM conformance statement, please click here.
DICOM=Digital Imaging and Communication in Medicine
OPTIS™ Imaging Systems can be easily connected to standard Boom monitors in the cath lab to display the OCT user interface on the boom monitor to view PCI information from the procedure table.
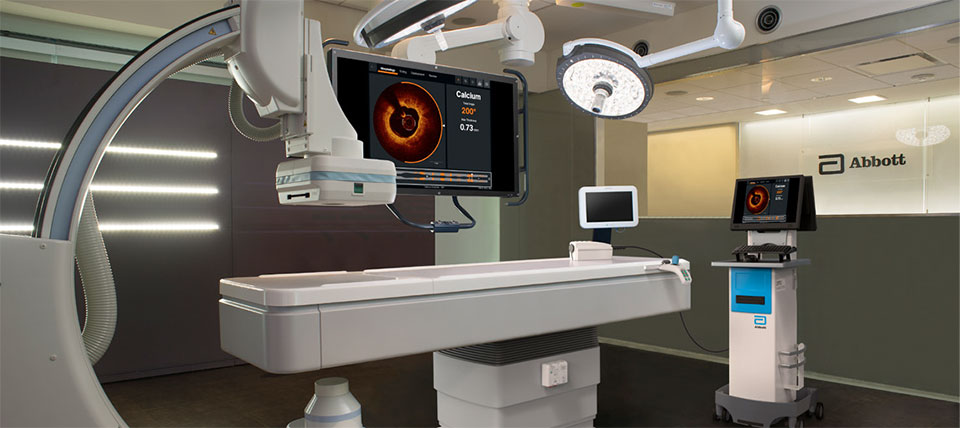
For more details on the DVI extender and Connectivity Box, see system components in the OPTIS™ Imaging Systems page.
The OPTIS ™ Imaging Systems can be integrated with the cath lab angio systems to display OCT and angio co-registration (ACR) on the same screen.

OCT and angio co-registration (ACR)
*RFR is available with AptiVue™ Imaging Software and Ultreon™ 1.0 Software.
Wi-Box™ AO Transmitter and OPTIS™ Imaging Systems and Software IFUs. Refer to Instructions For Use (IFU) for additional information.
MAT-2106761 v1.0

Indications The OPTIS™ Software and AptiVue™ E Series Software are intended to be used only with compatible OPTIS™ Imaging Systems.
The OPTIS™ Imaging System with a compatible Dragonfly™ Imaging Catheter is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The compatible Dragonfly™ Imaging Catheters are intended for use in vessels 2.0 to 3.5 mm in diameter. The compatible Dragonfly™ Imaging Catheters are not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ Imaging System is intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise and clinical judgment to determine if therapeutic intervention is indicated.
Contraindications: The OPTIS™ Integrated System and Mobile System with Software are contraindicated where introduction of any catheter would constitute a threat to patient safety. Contraindications include:
NOTE: The systems have no patient alarm functions. Do not use for cardiac monitoring.
Warnings:
Precautions:
MAT-2115909 v2.0

The Ultreon™ 1.0 Software is intended to be used only with compatible OPTIS™ Next Imaging Systems. The OPTIS™ Next Imaging System with a compatible Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure. The OPTIS™ Next Imaging System is intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment to determine if therapeutic intervention is indicated.
Contraindications: Use of the Ultreon™ 1.0 Software is contraindicated where introduction of any catheter would constitute a threat to patient safety.
Contraindications include:
Complications: The risks involved in vascular imaging include those associated with all catheterization procedures. The following complications may occur as a consequence of intravascular imaging and may necessitate additional medical treatment including surgical intervention.
Warnings:
Precautions:
MAT-2104193 v3.0
STAY CONNECTED