MAT-2403640 v1.0 | Item approved for U.S. use only. ©2024 Abbott. All Rights Reserved.
Assert-IQ™ Insertable Cardiac Monitor (ICM) is an advanced ICM, sometimes referred to as a a loop recorder, which provides efficient, accurate, and continuous patient monitoring.
This next gen ICM delivers meaningful value in the areas that are most important:
Whether you are a healthcare provider or a patient, Abbott’s Assert-IQ ICM offers an innovative solution for monitoring cardiac activity, delivering reliable data, and enabling better care decisions.5
Assert-IQ ICM is a Bluetooth®-enabled ICM that provides reliable, long-lasting cardiac monitoring with no compromises in features.
We offer two battery life options to suit different monitoring needs:
Assert-IQ 3+ | May be Preferred for Traditional Cardiac Monitoring.
≥ 3 years* may be preferred for more traditional reasons for monitoring, such as diagnosing syncope, palpitations, or detection of atrial fibrillation (AF) within cryptogenic stroke patients.
Assert-IQ EL+ | Longer Term Monitoring; May be Preferred for AF Management.
≥ 6 years* may be preferred for longer-term monitoring, such as those undergoing therapy and are at risk of developing further arrhythmias such as AF.
The Assert-IQ 3 ICM offers another 3-year battery life option that encapsulates only the essential and most valued ICM features.
Assert-IQ ICM is an innovative medical device designed to provide industry-leading accuracy for arrhythmia detection. Advanced algorithms build user confidence in transmitted data and provide reassurance to patients who need diagnosis by removing false positives. Each new enhancement for algorithm detection has been tested and proven to reduce false detections.1-2
Key Episode Technology provides flexibility for data management while maintaining time-to-diagnosis for patients. Key Episodes** are uniquely programmed for each arrhythmia type based on evaluation criteria that remove false positives versus a standard set of criteria for episode management. The feature can be toggled on/off as needed.
IQ insights provide trends and timelines of clinically relevant information to empower data driven decisions. Assert-IQ ICM provides a holistic view for patient management with new intelligent diagnostics:
Leading PVC detection algorithms offers the ability to capture consecutive events, including couplets and triplets.6
An ICM first.5, 7-11
Assess new patient factors to help adjudicate episodes.
Assess AFib Burden, PVCs, Activity Trends, and MORE!
Assert-IQ ICM offers simple and secure remote programming, allowing healthcare professionals to control any connected device remotely. Clinicians can adjust device settings, optimize performance, and eliminate unnecessary alerts or transmissions without requiring patients to visit the clinic physically.***
By leveraging the power of remote programming, healthcare providers can reduce clinic workflow burden and ensure data is transmitted unique to the patient.
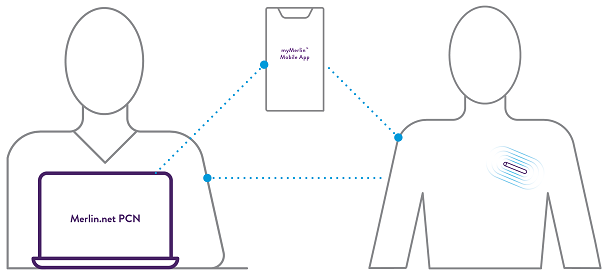
All parameters can be remotely programmed by the clinician.^
Sign up to hear about our technology, education opportunities, and more.
Stay up to date with recent news, product highlights, and case studies.

†As of 12.31.22. Reveal LINQ‡ User Manual, LINQ II‡ User Manual, Lux Dx‡ User Manual, Biomonitor III‡ User Manual, Biomonitor IIIm‡ User Manual.
*Data on File, Abbott - Report 90984075.
**Key Episodes is a feature of Merlin.net™ Patient Care Network.
***Available on DM5300/DM5500.
^Not applicable for Name, DOB, and reason for monitoring
Additional References
™ Indicates a trademark of the Abbott group of companies.
‡ Indicates a third-party trademark, which is property of its respective owner.
Bluetooth and Bluetooth logo are registered trademarks of Bluetooth SIG, Inc.
MAT-2303940 v6.0
STAY CONNECTED