MAT-2105201 v1.0
Benefit From Improved Lab Efficiency
We understand that things may not always go as planned during a procedure.
Unexpected access-site related complications can be costly and add to your schedule for the day.
You already spend your entire day helping patients. You and your team deserve to know when you'll be going home at the end of the day.
Commit to a consistent, predictable, and proven close.

Facilitate quick turnaround times between cases.

The confidence of over 30 years legacy and over 20 million closures.

The Perclose™ Difference
The PercloseTM ProStyleTM Suture-Mediated Closure and Repair (SMCR) System is the next generation design evolution of the proven and trusted Perclose ProGlideTM SMC System.1 It delivers a COMPREHENSIVE, VERSATILE and PROVEN solution for haemostasis management unrivalled by any other single offering.
That's why the Perclose™ Devices are the world's leading vascular closure system.†

Comprehensive
Breadth of Transfemoral Applications
Perclose™ Devices offer you broadest indications* on the market allowing for use across the widest range of transfemoral procedures.5-41
- Arterial Sheaths 5-21F1
(Max OD 26F)2 - Venous Sheaths 5-24F1
(Max OD 29F)2
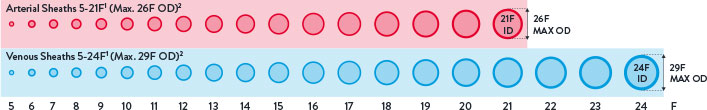
Max. OD 26F/0.340 inches/8.62 mm; Max. OD 29F/0.378 inches/9.59 mm. Data on file at Abbott.
Versatile
Keep Your Options Open
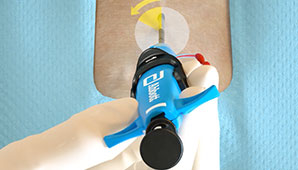
Before the Procedure
Customise your closing approach by deploying the sutures before the procedure (pre-close) or after the procedure, by using one or more devices per access site, or by rotating your angle of needle deployment to avoid any challenging anatomy.1
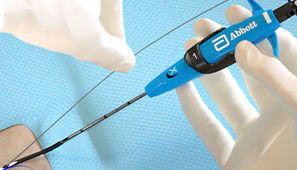
During the Procedure
Maintain guide wire access even after deployment of the Perclose™ sutures to keep all therapeutic options open.1
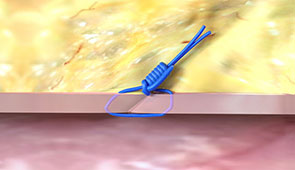
After the Procedure
Pull the sheath sooner due to no ACT-level requirements, and re-puncture the same access site immediately or in the near-term since Perclose™ Devices have no reaccess restrictions.1
Proven
Established Decades-Long Track Record
20,000,000+
closures and counting across 100+ countries worldwide2,3
> 30 Years
of Perclose™ Legacy as the pioneers of suture-mediated closure4
Years of clinical evidence featuring successful closure with Perclose™ products across a wide range of transfemoral procedures:
Percutaneous Coronary Intervention (PCI)5-7
Transcatheter Heart Valve Programs (TAVI, Mitral TEER, TMVR)8-18
Endovascular Aortic Repairs (PEVAR/TEVAR)18-22
Structural Heart Occluders (LAAO, PFO, ASD)23-25
EP Catheter Ablations and Leadless Pacemakers26-33
Vascular Interventional Radiology34-35
Interventional Neuroradiology (INR) and Neurovascular Interventions36-38
Mechanical Circulatory Support39-41
Getting Started on your Perclose™ Journey
Contact a Specialist
Identify which of the following transfemoral applications apply to your practice:
- Arterial access
- Venous access
- Small-bore access sites (≤8F)
- Large-bore access sites (>8F)
- Multiple access sites in the same vessel (EP)
Get Certified
Complete Abbott's official training program for the Perclose™ ProStyleTM SMCR System to learn the following:
- Single Device Deployment
- Double Perclose Technique for Large-Bore Closure
- The Pre-Close Technique vs. Post-Close Technique
- Tips and techniques for troubleshooting
Choose Efficiency
Make the decision to standardise your approach to haemostasis management by choosing a comprehensive, versatile, and proven vascular closure system that meets the majority of your transfemoral procedure needs.
Don't Just Close it. Perclose™ it.
PercloseTM ProStyleTM Suture-Mediated Closure and Repair System
Perclose™ ProStyle™ Suture-Mediated Closure and Repair (SMCR) System
The newest generation of the Perclose™ Family of Vascular Closure Systems designed with high-tensile strength needles for increased reliability and a more intuitive deployment experience compared to the older Perclose ProGlide™ SMC System.2

Other Haemostasis Management Solutions
Abbott also offers the following haemostasis management solutions for situations where Perclose™ Devices may not be preferred for the specific clinical situation.
StarClose SE™ Vascular Closure System (VCS)
The novel nitinol clip-based vascular closure system that achieves haemostasis for 5-6F arterial access sites for truly extravascular close.2,42
FemoStop™ Femoral Compression Systems
Offers hands-free compression of the femoral artery or vein to offer precise haemostasis management and patient comfort after transfemoral interventions.43

References:
✝ As of September 2024, per global market share data on file at Abbott.
*As compared to Angio-Seal‡, ExoSeal‡, FemoSeal‡, InClosure‡, MANTA‡, Mynx‡, PerQseal‡, Vascade‡, Velox CD‡, X-Seal‡. Data on file at Abbott.
Perclose™ Suture-Mediated Vascular Closure Systems
1. Perclose™ ProStyle™ SMCR System and Perclose ProGlide™ SMC System – Instructions for Use (IFU). Refer to IFU for additional information.
2. Data on file at Abbott.
3. July 2024 Finance Report. Data on file at Abbott.
4. On Nov. 8, 1993, the first (Perclose) patent was filed for the percutaneous suture vascular closure device.
Percutaneous Coronary Interventions (PCI)
5. Varis, E.; Gurbuz, D. C. Learning Curve of Perclose ProGlide Utilization During Percutaneous Coronary Intervention. Cureus, [s. l.], v. 15, n. 4, p. e38155, 2023.
6. Nikolsky, E. et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: A meta-analysis. Journal of the American College of Cardiology (JACC), [s. l.], v. 44, n. 6, p. 1200–1209, 2004.
7. Bhatt, D. L. et al. Successful “pre-closure” of 7Fr and 8Fr femoral arteriotomies with a 6Fr suture-based device (the Multicenter Interventional Closer Registry). The American journal of cardiology, [s. l.], v. 89, n. 6, p. 777–779, 2002.
Transcatheter Heart Valves
8. Drafts, B. C. et al. Comparison of outcomes with surgical cut-down versus percutaneous transfemoral transcatheter aortic valve replacement: TAVR transfemoral access comparisons between surgical cut-down and percutaneous approach. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions, [s. l.], v. 91, n. 7, p. 1354–1362, 2018.
9. McCabe, J. M. et al. Surgical Versus Percutaneous Femoral Access for Delivery of Large-Bore Cardiovascular Devices (from the PARTNER Trial). The American journal of cardiology, [s. l.], v. 117, n. 10, p. 1643–1650, 2016.
10. Dencker, D. et al. Frequency and Effect of Access-Related Vascular Injury and Subsequent Vascular Intervention After Transcatheter Aortic Valve Replacement. The American journal of cardiology, [s. l.], v. 118, n. 8, p. 1244–1250, 2016.
11. Barbash, I. M. et al. Comparison of vascular closure devices for access site closure after transfemoral aortic valve implantation. European heart journal, [s. l.], v. 36, n. 47, p. 3370–3379, 2015.
12. Dimitriadis, Z. et al. Impact of closure devices on vascular complication and mortality rates in TAVI procedures. International Journal of Cardiology, [s. l.], v. 241, p. 133–137, 2017.
13. Barbash, I. M. et al. Comparison of MANTA versus Perclose Prostyle large-bore vascular closure devices during transcatheter aortic valve implantation. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions, [s. l.], v. 103, n. 1, p. 160–168, 2024.
14. Hoffmann P et al. Access site complications after transfemoral aortic valve implantation - a comparison of Manta and ProGlide. CVIR Endovascular, [s. l.], v. 1, n. 1, p. 1–8, 2018.
15. Mohammed, M. et al. Preclosure of large bore venous access sites in patients undergoing transcatheter mitral replacement and repair. Catheterization & Cardiovascular Interventions, [s. l.], v. 100, n. 1, p. 163–168, 2022. (TEER)
16. Geis, N. A. et al. Feasibility and clinical benefit of a suture-mediated closure device for femoral vein access after percutaneous edge-to-edge mitral valve repair. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology, [s. l.], v. 10, n. 11, p. 1346–1353, 2015. (TEER)
17. Kar S, Hermiller J et al. The use of Perclose ProGlide SMC System for Venous Access-Site Closure up to 24F Sheaths. CRT 2018 Poster.
18. Schneider, D. B. et al. Clinical and economic outcomes of ProGlide compared with surgical repair of large bore arterial access. Journal of comparative effectiveness research, [s. l.], v. 8, n. 16, p. 1381–1392, 2019.
Endovascular Aortic Repair (PEVAR/ TEVAR)
19. Pratesi, G. et al. Italian Percutaneous EVAR (IPER) Registry: outcomes of 2381 percutaneous femoral access sites’ closure for aortic stent-graft. The Journal of cardiovascular surgery, [s. l.], v. 56, n. 6, p. 889–898, 2015.
20. Nelson, P. R. et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). Journal of Vascular Surgery, [s. l.], v. 59, n. 5, p. 1181–1193, 2014.
21. Krajcer, Z.; Ramaiah, V.; Huetter, M. Fast-track endovascular aneurysm repair: rationale and design of the multicenter Least Invasive Fast-Track EVAR (LIFE) registry. BMC cardiovascular disorders, [s. l.], v. 15, p. 174, 2015. 8. Saadi, E. K. et al. Totally Percutaneous Access Using Perclose Proglide for Endovascular Treatment of Aortic Diseases. Brazilian Journal of Cardiovascular Surgery, [s. l.], v. 32, n. 1, 2017.
22. Roche-Nagle, G.; Hazel, M.; Rajan, D. K. Financial Impact of PEVAR Compared With Standard Endovascular Repair in Canadian Hospitals. Canadian Association of Radiologists Journal, [s. l.], v. 69, n. 2, p. 215–219, 2018.
Structural Heart Occluders (LAAO, PFO, ASD)
23. Lodhi, H. et al. Comparison of Figure-of-Eight Suture and Perclose ProGlide Suture-Mediated Closure in Large Bore Venous Access Hemostasis: A Randomized Controlled Trial. The American journal of cardiology, [s. l.], v. 209, p. 181–183, 2023.
24. Steiner, K. et al. Same-day discharge after percutaneous closure of persistent foramen ovale using intracardiac echocardiography and the Gore Septal Occluder. Frontiers in cardiovascular medicine, [s. l.], v. 11, p. 1408543, 2024.
25. Han, X. et al. Perclose ProGlide devices simplified the removal of the femoral venous cannulas for the transcatheter closure of atrial septal defect: A single-center retrospective study. Vascular Investigation & Therapy, [s. l.], v. 5, n. 2, p. 31–36, 2022.
Cardiac Ablations (RF, Cryo, PFA)
26. Fabbricatore, D. et al. Ambulatory pulmonary vein isolation workflow using the Perclose ProGlide™ suture-mediated vascular closure device: the PRO-PVI study. EP: Europace, [s. l.], v. 25, n. 4, p. 1361–1368, 2023.
27. Kiani, S. et al. Percutaneous Vascular Closure Compared With Manual Compression in Atrial Fibrillation Ablation. JACC. Clinical electrophysiology, [s. l.], v. 8, n. 6, p. 803–805, 2022.
28. Verma, S. et al, Feasibility and Safety of Same Day Discharge for Patients Undergoing Atrial Fibrillation (AF) Ablation in a Community Hospital Setting. HRS 2020 Science Online, May 2020.
29. Sun, J.-Y. et al. Feasibility and clinical benefits of the double-ProGlide technique for hemostasis after cryoballoon atrial fibrillation ablation with uninterrupted oral anticoagulants. Journal of geriatric cardiology : JGC, [s. l.], v. 20, n. 4, p. 268–275, 2023.
30. Tilz, R. R. et al. Venous vascular closure system vs. figure-of-eight suture following atrial fibrillation ablation: the STYLE-AF Study. EP: Europace, [s. l.], v. 26, n. 5, p. 1–12, 2024.
31. Kiani, S. et al. Costs, efficiency, and patient-reported outcomes associated with suture-mediated percutaneous closure for atrial fibrillation ablation: Secondary analysis of a randomized clinical trial. Journal of cardiovascular electrophysiology, [s. l.], 2024.
Leadless Pacemakers
32. Deshmukh, A. et al. Double ProGlide preclose technique for vascular access closure after leadless pacemaker implantation. Journal of Interventional Cardiac Electrophysiology, [s. l.], v. 63, n. 2, p. 341–343, 2022.
33. Rodriguez-Taveras, J. et al. Double Perclose increases the efficiency of leadless pacemaker implantation: A propensity score–matched analysis. Heart Rhythm O2, [s. l.], v. 5, n. 10, p. 750–753, 2024.
Vascular Interventional Radiology
34. Jensen, J. et al. The Inflammatory Response to Femoral Arterial Closure Devices: A Randomized Comparison Among FemoStop, AngioSeal, and Perclose. CardioVascular & Interventional Radiology, [s. l.], v. 31, n. 4, p. 751–755, 2008.
35. Henk, C. B. et al. ‘The Closer’-percutaneous vascular suture device: evaluation of safety and performance in neuroangiography. European Journal of Radiology, [s. l.], v. 48, n. 3, p. 237–243, 2003.
Neurovascular Interventions & Interventional Neuroradiology
36. Layton, K. F. et al. Use of the Perclose ProGlide device with the 9 French Merci retrieval system. Neuroradiology, [s. l.], v. 48, n. 5, p. 324–326, 2006.
37. Khaghany, K. et al. Efficacy and safety of the perclose closer s device after neurointerventional procedures: prospective study and literature review. AJNR. American journal of neuroradiology, [s. l.], v. 26, n. 6, p. 1420–1424, 2005.
38. Rajan, J. E. et al. Prospective Evaluation of Factors Affecting the Safety and Efficacy of Perclose Proglide Vascular Closure Device in Neurovascular Interventions. Neurology India, [s. l.], v. 67, n. 5, p. 1305–1309, 2019.
Mechanical Circulatory Support
39. Lata, K. et al. Pre-close technique of percutaneous closure for delayed hemostasis of large-bore femoral sheaths. Journal of Interventional Cardiology, [s. l.], v. 31, n. 4, p. 504–510, 2018.
40. Unoki, T. et al. Efficacy and safety of post-closure technique using Perclose ProGlide/ProStyle device for large-bore mechanical circulatory support access sites. Cardiovascular Revascularization Medicine, [s. l.], v. 62.
41. Sun, G. et al. Outcomes comparison between percutaneous decannulation with perclose ProGlide and surgical decannulation of veno-arterial extracorporeal membrane oxygenation. Perfusion, [s. l.], p. 1, 2023.
Other Haemostasis Management Solutions IFUs
42. StarClose SE™ VCS System - Instructions for Use (IFU). Refer to IFU for additional information.
43. FemoStop™ II Plus Femoral Compression System - Instructions for Use (IFU). Refer to IFU for additional information.
MAT-2409380 v2.0

