Amplatzer™ Septal Occluders are the standard of care for minimally invasive atrial septal defect (ASD) closure.1,2 These double-disc occluders are comprised of nitinol mesh and polyester material. They are designed to securely appose the septal wall on each side of the defect and create a platform for tissue in-growth after implantation.
Atrial septal defects come in a variety of shapes and sizes. There can often be multiple, or fenestrated, ASDs. Abbott offers two percutaneous, transcatheter occluders for different types of atrial septal defects.
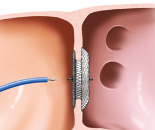
The Amplatzer™ Septal Occluder has a wide waist that centers the device and fills the ASD for optimal occlusion. The transcatheter delivery makes it the proven standard of care for the occlusion of ASDs.
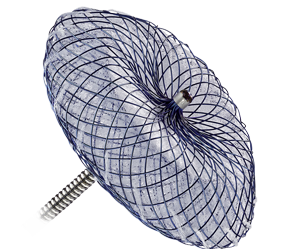
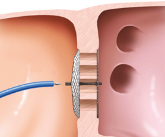
The Amplatzer™ Cribriform Occluder has a narrow waist, which allows for placement through a central defect, and matched disc diameters that maximize coverage of multiple fenestrations.

Left: The waist of the Amplatzer Septal Occluder fills the defect for optimal occlusion. Right: The Amplatzer Cribriform Multi-Fenestrated Septal Occluder enables occlusion of the defect by covering the fenestrations with a single device.
| Model/Reorder Number | Waist Diameter (mm) | Waist Width (mm) | Right Atrial Disc Diameter (mm) | Left Atrial Disc Diameter (mm) |
|---|---|---|---|---|
| 9-ASD-004 | 4 | 3 | 12 | 16 |
| 9-ASD-005 | 5 | 3 | 13 | 17 |
| 9-ASD-006 | 6 | 3 | 14 | 18 |
| 9-ASD-007 | 7 | 3 | 15 | 19 |
| 9-ASD-008 | 8 | 3 | 16 | 20 |
| 9-ASD-009 | 9 | 3 | 17 | 21 |
| 9-ASD-010 | 10 | 3 | 18 | 22 |
| 9-ASD-011 | 11 | 4 | 21 | 25 |
| 9-ASD-012 | 12 | 4 | 22 | 26 |
| 9-ASD-013 | 13 | 4 | 23 | 27 |
| 9-ASD-014 | 14 | 4 | 24 | 28 |
| 9-ASD-015 | 15 | 4 | 25 | 29 |
| 9-ASD-016 | 16 | 4 | 26 | 30 |
| 9-ASD-017 | 17 | 4 | 27 | 31 |
| 9-ASD-018 | 18 | 4 | 28 | 32 |
| 9-ASD-019 | 19 | 4 | 29 | 33 |
| 9-ASD-020 | 20 | 4 | 30 | 34 |
| 9-ASD-022 | 22 | 4 | 32 | 36 |
| 9-ASD-024 | 24 | 4 | 34 | 38 |
| 9-ASD-026 | 26 | 4 | 36 | 40 |
| 9-ASD-028 | 28 | 4 | 38 | 42 |
| 9-ASD-030 | 30 | 4 | 40 | 44 |
| 9-ASD-032 | 32 | 4 | 42 | 46 |
| 9-ASD-034 | 34 | 4 | 44 | 50 |
| 9-ASD-036 | 36 | 4 | 46 | 52 |
| 9-ASD-038 | 38 | 4 | 48 | 54 |
| Model/Reorder Number | Right & Left Atrium Disc Diameter (mm) | Waist Width (mm) |
|---|---|---|
| 9-ASD-MF-018 | 18 | 3 |
| 9-ASD-MF-025 | 25 | 3 |
| 9-ASD-MF-030 | 30 | 3 |
| 9-ASD-MF-035 | 35 | 3 |
MAT-2006763 v2.0
STAY CONNECTED